
Normal Insulin
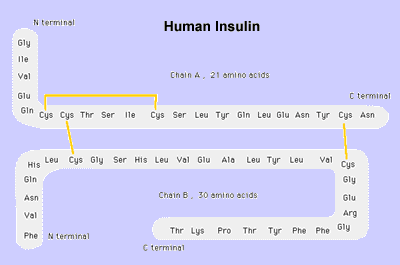
The normal insulin molecule is composed of two peptide chains referred to as the A chain and B chain.
Insulin Analogues
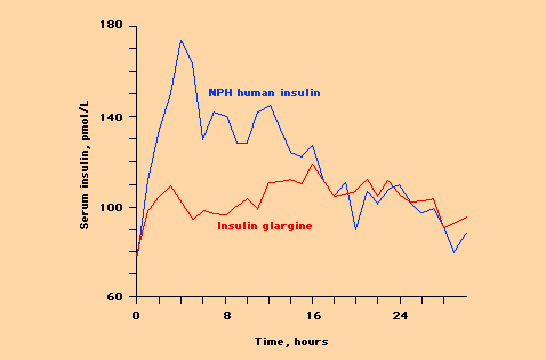
The insulin molecule has been modified in several ways so as to change the speed from which it is absorbed from the subcutaneous tissues. This allows these subcutaneously administered insulin analogues to better mimic the secretion pattern of the pancreas which in a normal person without diabetes secretes sufficient amounts of insulin directly into the blood stream. Several of these analogues are currently available. Insulin glargine is a basal (long-acting analogue) which has been modified to have a longer duration of action with minimal peak effect. Insulin lispro and insulin aspart have been modified to have the exact opposite effect. These short acting analogues are rapidly absorbed with a very early peak and very short duration of action.
Insulin Glargine (Lantus)
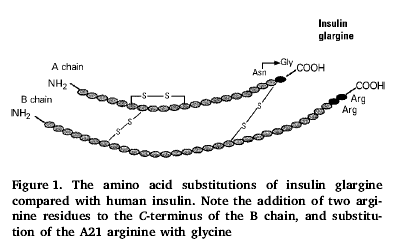
· Addition of 2 arginine residues to the C-terminus of the b-chain.
· Change of the 21 amino acid of a-chain from arginine to glycine.
· Substitution with glycine and arginine residues gave rise to the name insulin ‘glargine’.
· The 2 arginine residues added to the B-chain result in an insulin which is soluble in an acidic pH 4.0-5.0 of the injection medium but precipitates once injected into the subcutaneous tissues where the pH 7.4 is physiological.
· Less soluble insulin is absorbed slower but is therefore more susceptible to degradation before it is absorbed.
· The A21 arginine to glycine change retards this degradation.
Insulin Lispro (Humalog)
·
Insulin
molecules have a tendency to form dimers in solution due to hydrogen-bonding
between the C-termini of B chains. Additionally, in the presence of zinc ions,
insulin dimers associate into hexamers.
· In insulin lispro there is a reversal of the order of the 28th and 29th amino acids of the Beta-chain, where normally Proline is 28th Lysine is 29th.
·
This modification does not alter receptor binding, but minimizes
the tendency to form dimers and hexamers.
Insulin Aspart (NovoRapid)
· 28th amino acid on B-chain proline has been substituted with aspartic acid.
· This change reduces the tendency of the molecules to self-associate. Therefore, the speed of absorption into the bloodstream after subcutaneous injection is greatly increased.
Insulin Detemimr (NN304 by Novo Nordisc)
Removal of the terminal 30th amino-acid of the Beta-chain (a threonine) and the addition of a C14 fatty acid to the 29th amino-acid.
Insulin Detemir is 98% reversibly bound to free fatty acid binding sites on albumin in plasma and interstitial fluid. This unique mechanism of albumin binding prolongs its duration of action, and contrasts with existing long-acting insulins whose duration of action is dependent on the rate of dissociation of various sized crystals at the subcutaneous site. The mechanism of an insulin analogue binding to albumin provides a depot of insulin in the blood rather than the subcutaneous tissue.
Insulin Determir is currently in Phase III studies and is not yet available.